Conference Reviewed | Guangdong Medical Association Urology Calculi Special Academic Symposium(GDMA2025) was successfully held, highlighting the innovative hard power of Endoso Life
1970-01-01 08:33:45

The conference attracted 120 urologists from 36 countries and nearly 30 top international experts to give lectures. At the conference site, the booth of Endoso Life was constantly visited by visitors.

The reusable 7.5F ureteroscope and digital cystoscope exhibited by Endoso this time have both been affirmed and praised by the experts present at the conference. Experts believe that the Endoso multi-purpose cystoscope has an extremely slender body (2.5mm), and its light handle is easy to handle. It is not tiring to support for a long time during the operation. Even doctors with less experience can master it easily. It is an indispensable tool for urologists.

The Endoso digital ureteroscope, with its fine and flexible body design, can gently pass through the ureter and renal pelvis, minimizing damage to the mucosa to the greatest extent.
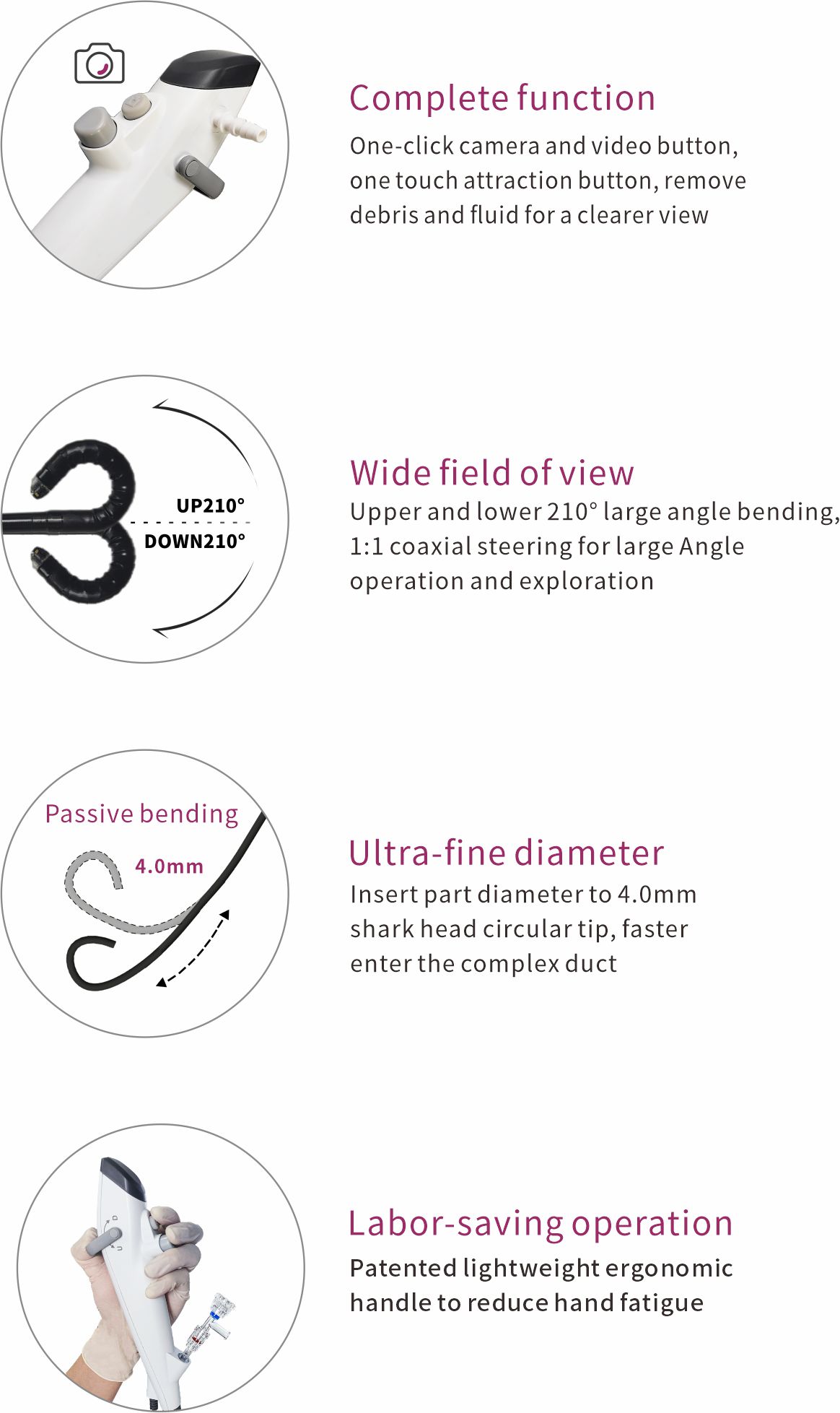
The up and down bending ability of up to 330 degrees at the end of the lens makes it more flexible and convenient when observing and treating stones in the renal pelvis and calyces.
The conference also showcased a variety of highly challenging minimally invasive surgeries, including urinary calculi surgery, urinary tumor surgery and urinary tract reconstruction surgery, and was simultaneously live-streamed online.
 In the future, Endoso Life will maintain its original intention, adhere to the demands of doctors and patients in the first place, adhere to independent research and development, focus on clinical, and meet clinical and patient needs with high-quality products and services
In the future, Endoso Life will maintain its original intention, adhere to the demands of doctors and patients in the first place, adhere to independent research and development, focus on clinical, and meet clinical and patient needs with high-quality products and services